 Research Article
Research Article
Short-Term Efficay and Tolerability of The Antispasmodic Methocarbamol in Acute Low Back Pain – Results of Metabo, A Patient-Level Pooled Re- Analysis of Original Data from Two Double-Blind Randomized and Placebo-Controlled Trials
Michael A Überall*, Michael A Küster, Philipp Müller-Schwefe and Gerhard H H Müller Schwefe
1Institute of Neurological Sciences, IFNAP, Germany
2Interdisciplinary Pain Center Bonn, Bad Godesberg, Germany
3Interdisciplinary Pain and Palliative Care Center Göppingen, Germany
4Interdisciplinary Pain and Palliative Care Center Göppingen, Germany
Michael A. Überall, MD, Institute of Neurological Sciences - IFNAP, Nordostpark 51, 90411 Nürnberg, Germany E-Mail: Phone: ++49 911 21773760 Fax: ++49 911 21773761
Received Date:November 20, 2023; Published Date:November 24, 2023
Abstract
Objective:To evaluate the short-term 72-hr. analgesic efficacy and safety/tolerability of the non- benzodiazepine antispasmodic methocarbamol (MET) in patients with acute low back pain (aLBP).
Method:Patient-level pooled re-analysis of depersonalized raw data from two double-blind placebo- controlled trials (EUPAS: 50070). Primary efficacy analysis based on a composite endpoint consisting of five response criteria (pain intensity, finger-to-floor distance, Lasègue sign, overall mobility, and interference with night sleep). Safety/tolerability assessment based on adverse drug reactions (ADRs), and premature treatment discontinuations.
Results:In total, data from 359 patients (age 46.5±10.6/46.1±11.4 years; 59.3/59.9% female), of whom 177 received a monotherapy with MET and 182 with PLC, were extracted from the original case report forms. Overall, 14/6 ADRs were reported in 10/4 (5.6/2.2%) patients with MET/ PLC (p=0.105). Discontinuation rates (either due to ADRs, insufficient analgesia or patient request) were significantly lower for MET vs. PLC (11.9% vs. 31.9%; p<0.001). Treatment with MET was followed by a significantly higher percentage of patients who reached the primary endpoint vs. PLC: 57.6% vs. 31.3%; p<0.001; effect size: 0.265; NNT: 3.9; OR: 2.982 (95%-CI: 1.935-4.596), RR: 1.840 (95%-CI: 1.435-2.361), and biometrical analyses confirmed the superiority of MET vs. PLC. Subsequent analyses confirmed significantly greater effect rates for iv vs. po MET (p=0.009).
Conclusion:The 72-hr. short-term treatment of aLBP with the non-benzodiazepine antispasmodic MET proved superior effective compared to PLC. Treatment results achieved by iv MET were significantly stronger than those after po administration.
Keywords:Methocarbamol; Acute Low Back Pain; Antispasmodic; Double-Blind Randomized Trial; Short-Term Treatment; Superiority
Transparency
Declaration of financial/other relationships
The concept for this re-analysis was developed by M.A.U. at the Institute of Neurological Sciences (IFNAP) on behalf of the German Pain Association (Deutsche Gesellschaft für Schmerzmedizin, DGS) and the German Pain League (Deutsche Schmerzliga, DSL). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. All authors are physicians and independent of any significant/relevant financial or other relationship to the manufacturer of the product under evaluation, except for minor reimbursements for occasional lecture or consulting fees. M.A.U. is honorary member of the management board of the German Pain Association (vice-president) and the German Pain League (president). M.A.K. is past- vice-president of the German Pain Association. G.H.H. M.-S. is past-president of the German Pain Association.
Authors contribution
All authors were involved in literature research, screening and eligibility evaluation as well as drafting the article or revising it critically for important intellectual content, and all authors read and approved the final manuscript to be published. M.A.U. takes responsibility for the integrity of the work as a whole, from inception to the finished article and affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Background
Acute low back pain (aLBP) is one of the most common reasons for adults to consult a physician, visit an emergency department or take pain medication. In 2020, aLBP affected 619 million people worldwide and evolved into the leading cause of years lived with disability (YLD) in industrialized countries - especially those in Western Europe [1,2]. Although most cases of aLBP resolve spontaneously (i.e., with or without medication or nonpharmacological counter measures) within a few days, 31% of all patients report persistent/recurrent symptoms in the first six months. 25-62% experience a recurrence within one to two years (with up to 33% suffering from moderate and 15% severe to very severe pain), [3-5] and up to 60% of patients are at risk for developing chronic LBP [6,7]. Since the majority of cases (i.e., those more than 90% with so-called non-specific low back pain) present no specific disease or structural reason to explain their pain, [8] it is often difficult to initiate an appropriate (causative) pain therapy. Clinicians must therefore assess which treatment is best suited to alleviate the acute symptoms and prevent possible recurrences, given the individual history, the presence of comorbid conditions, clinical findings, and severity of the acute events.
Irrespective of the underlying cause, the majority of patients with aLBP report movement-dependent pain and show a relevant and clinically reproducible detectable movement restriction in the lumbar region on physical examination [e.g., Schober´s test (ST), finger-to-floor distance (FFD), Lasegue sign (LS), etc.] - which point to some (direct or indirect) pathophysiological involvement of muscular structures. Although these findings would justify the use of antispasmodics for the treatment of aLBP from a mechanistic point of view, current guidelines do not differentiate between various causes of pain and list antispasmodics at best as second line options or sometimes do not even recommend their use at all (mostly justified by their unclear mechanisms of action, various tolerability problems, insufficient scientific evidence, and their - in view of the high spontaneous remission in the first few days - low efficacy).
Recently, a very specific peripheral effect site (in the area of the muscle spindles) as well as a mode of action (block of muscular Nav 1.4 channels) has been demonstrated for the antispasmodic methocarbamol (MET) [9], what distinguishes this guaiacol glyceryl ether from other muscle relaxants, which are generally assumed to exert benzodiazepine- or atropine-like nervous system effects either central on spinal-motoneurons or peripheral on the nerve muscle junction and whose practical use is accompanied by frequent (and often serious or at least treatment limiting) adverse drug reactions (ADRs).
Originally developed in the early 1950s as a treatment for muscle spasticity and associated pain, MET is available as tablet as well as iv/im-injection and approved as prescription add-on medicine to rest, physio- and physical therapy as well as other countermeasures in the United States and several European countries to alleviate physical discomfort resulting from acute, painful diseases of the musculoskeletal system, such as aLBP.
In everyday use, MET has proven to be a well-tolerated alternative to other antispasmodics, [10, 11] but there is a lack of transparently published data from double-blind randomized placebo-controlled trials (RCTs) - which is why its use for the treatment of aLBP is still neither mentioned nor recommended in current guidelines. However, there are two RCTs on MET in that indication that were conducted at the request of the German Federal Institute for Drugs and Medical Devices (BfArM) for the purpose of subsequent authorization respectively the continuation of authorization. Both RCTs were conducted at the beginning of the 21st century and designed to confirm effect, safety, and tolerability of both, the intravenous (iv) parenteral and the oral (po) dosage forms vs. placebo (PLC) for the short-term treatment of aLBP and fulfilled the requirements of the approval authority. Unfortunately, the results of these two trials have so far only been published in two German-language medical journals and not internationally in appropriate peer-reviewed journals [11, 12].
Objective
The primary objective of this analysis was to evaluate the shortterm/ 72-hour efficacy, safety and tolerability of MET compared to PLC in adults with aLBP based on a completely new, patient-level pooled re-analysis of aggregated raw data from two placebocontrolled clinical [11, 12]. The secondary objectives of this study focused on the differential response to the intravenous (iv) versus oral (po) administrations of the active study medication MET.
Materials And Methods
Data source
This study used only raw data reported in and extracted from the integrated biometrical reports and the corresponding original case report forms (CRFs) of two double-blind, randomized, placebocontrolled clinical trials [11, 12]. Both studies were conducted in Germany from 2002 to 2003 to evaluate the short-term efficacy and tolerability of iv and oral methocarbamol compared to placebo (studies BST-01 and BST-02) over three respective seven days. Both studies were planned and conducted at the same time and shared many similarities that made it possible to extract and summarize the data for the purposes of the present re-analysis.
Treatment effect evaluation period
Due to differences in the realized study duration (3 days for iv and 7 days for po MET), the duration for the intended comparative effectiveness evaluation vs. PLC was 3 days.
Study parameters
From the variables documented in the above-mentioned clinical studies, only those data were extracted that were necessary to conduct the analyses described in the statistical analysis plan of this study. Age, gender, height, weight, and body mass index (BMI) as well as documented comorbidities were extracted to review possible outcome-relevant between-group differences of the study patient’s prior medication. Pain intensity (PI; mm VAS), fingerto- floor distance (FFD; cm), pain-related movement restrictions (MOB; 4-point Likert scale: none, mild, moderate, severe), Lasègue sign (LS; positive, negative), pain-related sleep disturbances (PSD; 3-point Likert scale: none, occasional, two or more awakenings per night) were transferred before initiation of study medication and at the end of day 1-3 of treatment to assess efficacy. In addition, information if/when patients became pain free and/or experienced what type of adverse events and/or discontinued study medication as well as recapitulative information on the patient-reported general assessment of the physical limitations caused by aLBP (5-point Likert scale: none, mild, moderate severe, very severe) and the global effectiveness of the study medication (5-point Likert scale: very good, good, moderate, poor, very poor) at the end of day 3 were selected to gain insight into the short-term benefit-risk profile of the study medication.
Statistical analyses
As this study was based on already existing raw data from two double-blind, randomized, placebo- controlled trials, there were no additional specific inclusion and exclusion criteria for this study. All disease- and treatment-relevant data necessary for this re-analysis were extracted from the depersonalized original CRFs of the two studies BST-01 and BST-02, transferred to a new database for the purpose of this analysis and stratified according to the drugs investigated (cohort MET: methocarbamol; cohort PLC: placebo) and the dosage form (iv or po). Data analyses were performed on the full set of anonymized raw data extracts using a modified intent-to-treat approach (i.e., patients who had a complete baseline documentation with respect to the data necessary for the completion of this re-analysis and who took/ received at least one dose of the study treatments were evaluated). Missing data beyond baseline documentation were imputed using the last observation carried forward (LOCF) approach if necessary to guarantee 100% complete data for subsequent analyses. Results were summarized descriptively for baseline and end of day 1 to 3 data as well as absolute and relative changes from baseline using appropriate summary statistics and/or frequency distributions. Descriptive statistical analyses were performed as follows: for continuous variables, descriptive statistics were summarized by the number of patients (n), mean, standard deviation (SD), 95% confidence interval (95% CI) of the mean, median and range (minimum - maximum); for categorical and ordinal variables, data were summarized as frequency count (n) and percentage (%) of participants in each category. For comparisons between groups of 2x2 dichotomous/binomial contingency tables, the chi-square test, and for multinomial categorical variables, the Pearson chi-square test was used. Between-group comparisons of continuously scaled variables were performed depending on the data distribution using either the student´s t-test (for normally distributed data) or Wilcoxon’s rank sum test (for non-normal distributions). In addition, odds ratios (OR), risk ratio (RR) including the corresponding 95% confidence intervals (CI), and the numbers needed to treat/ harm (NNT/H) were calculated. Graphical effect estimates were presented as forest plots using the Mantel- Haenszel (M-H) analysis method (random model). Corresponding effect sizes (ES; Cohen’s d or PHI correlation) were calculated dependent of data category. All statistical tests were performed with a two-sided significance level of 0.05. Test results were reported as concrete significance values (p-values) up to a level of 0.001, lower p-values were assessed as “≤0.001”. As all comparisons except the primary endpoint were classified as exploratory, the significance levels were not adjusted for multiple testing. Meta-analytical calculations were carried out using the Review Manager program version 5.4; [13] further biometric analyses were performed using PASW Statistics (version 18.0); tables and charts (if required) were created/rendered using Microsoft Excel [14].
Primary efficacy endpoint
The proportion of patients classified as responders (as defined below) was the primary efficacy outcome variable and was compared between the two treatments (methocarbamol and placebo) regardless of route of administration. For the purposes of this analysis, a responder was defined as a patient who met all of the following five response criteria at the end of day 3 after initiation of study medication: Pain intensity (PI): proportion of patients with a clinically relevant/significant pain intensity improvement (i.e., either ≤-20mm VAS and/or ≤-50 percent) vs. baseline. Finger-floor distance (FFD): proportion of patients with a clinically relevant/significant FTF improvement (i.e., either ≤-15cm or ≤-50 percent) vs. baseline. Pain-related mobility restrictions (MOB): proportion of patients who reported either no or only mild pain-related restriction in their lumbar mobility. Lasègue sign (LS): proportion of patients with a negative Lasègue sign. Pain-related sleep disturbances (PSD): proportion of patients without sleep disturbances.
Secondary efficacy endpoints
The proportion of patients who became completely pain free until end of day 3 with study medication, the individual changes seen with the five criteria that made up the composite response definition, as well as the severity of aLBP complaints, the patientreported global effectiveness of study medication, and differential effects of study drug administration (iv vs. po) were analyzed as secondary efficacy endpoints.
Safety and tolerability endpoints
Safety was assessed by summarizing and analyzing the frequency and spectrum of adverse events (AEs), adverse drug reactions (ADRs), the number of patients with ADRs and ADRrelated treatment discontinuations.
For the purpose of this study, an AE was defined as any medically relevant event reported by patients during study participation within the first 72 hours of treatment initiation, regardless of its possible relation to the study medication under investigation. ADRs were defined as adverse events reported within the 72- hr. evaluation period for which the responsible study physician has documented at least a possible connection with the study medication in the original CRFs.
Primary endpoint analysis
For the calculation of the primary efficacy endpoint, a
superiority analysis was performed and the superiority of MET
over PLC was confirmed:
a) if the proportion of MET patients on methocarbamol who
met the primary efficacy endpoint (i.e., had positive results
for all five response criteria) was significantly higher than the
proportion of PLC-patients (p-value < 0.05), and
b) if the effect size analysis for this difference confirmed a
clinically relevant (>0.2) difference between the two treatment
cohorts in favor of MET versus PLC, and
c) if the lower limit of the 95% confidence interval of the
responder rate for the primary efficacy endpoint or MET was
above the upper limit of the 95% confidence interval for PLC,
and
d) if the 72-hour treatment discontinuation rate due to
adverse drug reactions, inadequate efficacy and/or patient
desire documented for MET was significantly lower than that for PLC.
Ethics
Both studies (on which this re-analysis is based) were originally reviewed by the ethics committees responsible and their implementation was approved. As this study exclusively used already available raw data in depersonalized form, there was no legal need for a formal new medical or data protection ethics review. The study design of the present re-analysis was reviewed and approved by the executive board of the German Pain Association (Germany’s largest healthcare organization for pain patients). The suitability of all protective measures for safeguarding the interests and personal rights of former study patients was reviewed and the final study concept approved by the board of the German Pain League (Germany’s largest advocacy organization for people with acute and chronic pain). The concept for this study was registered in the ENCEPP registry of the European Medicines Agency (EMA) before the start of data extraction and biometric analyses and thus made public (EUPAS: 50070).
Results
Patient population and demographics
With reference to the raw data provided via the integrated reports of the clinical trials BST-01 and BST-02, information on a total of 359 patients (n=202 from BST-01 for oral use and n=157 from BST-02 for intravenous use; n=177 for MET and n=182 for PLC) could be extracted and were used for the present analysis. All patients received at least one dose of the original study medication and their documented data thus fulfilled the requirements for extraction and biometric use in this re-analysis (Figure 1). A total of 229 patients (63.8%) – 120/109 with MET/ PLC (67.8/59.9%)– received their study medication over the entire evaluation period. 130 patients (36.2%) – 57/73 with MET/PLC (32.2/40.1%) – discontinued treatment prematurely (p=0.122), most frequently due to insufficient analgesic efficacy (5.1/26.4%; p<0.001), complete pain relief (20.3/8.2%; p=0.001), other reasons (5.6/2.2%; p=0.461) and ADRs (6.8/4.9%; p=0.511). On average, study patients were 46.5±10.6/46.1±11.4 years old and 59.3/59.9% were female (Table 1). Comorbid conditions were prevalent and affected 32.8/39.6% of MET/PLC patients. The most frequently documented health problems related to the cardiovascular system (24.9/26.9%), metabolism (7.9/7.7%), digestive organs (6.2/8.8%) and the musculoskeletal system (6.8/5.5%). 10.7/8.8% of the study patients documented a history of repeated aLBP (p=0.535).
Table 1:Key demographic characteristics
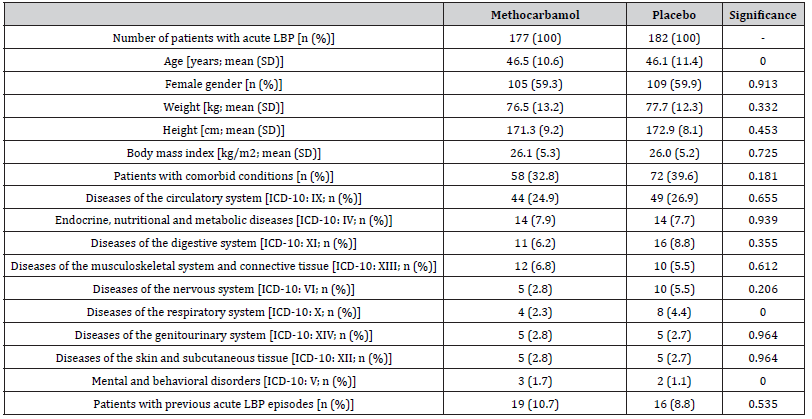
Abbreviations used: LBP: low back pain; n: number of patients; % percent; SD: standard deviation; kg: kilogram; cm: centimeter; m2: square meter; ICD-10: international classification of diseases version 10.
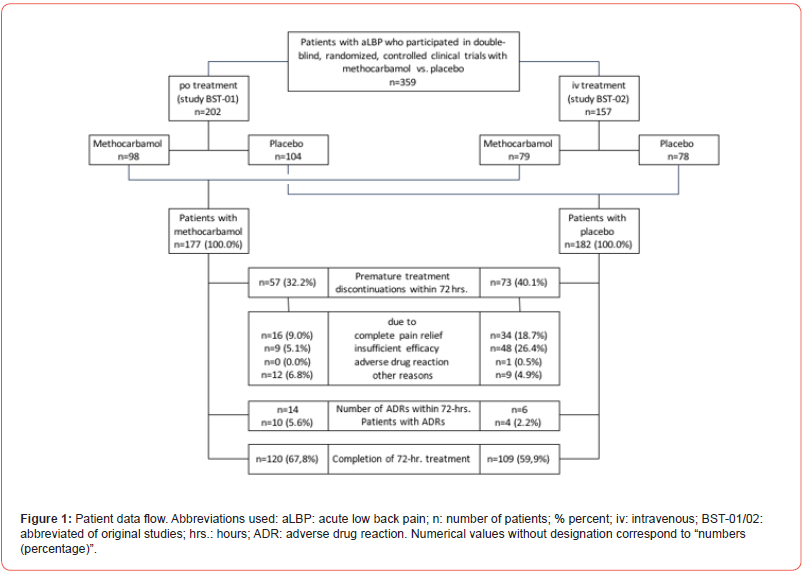
Study medication
In both studies, patients were assigned to one of the two treatment groups (MET or PLC) on the basis of a 1:1 randomization. MET treatment was administered either po (study BST-01; 1,500mg TID) or iv (study BST-02; 1,000mg BID); PLC therapy was administered with matching decoy preparations.
Treatment effects
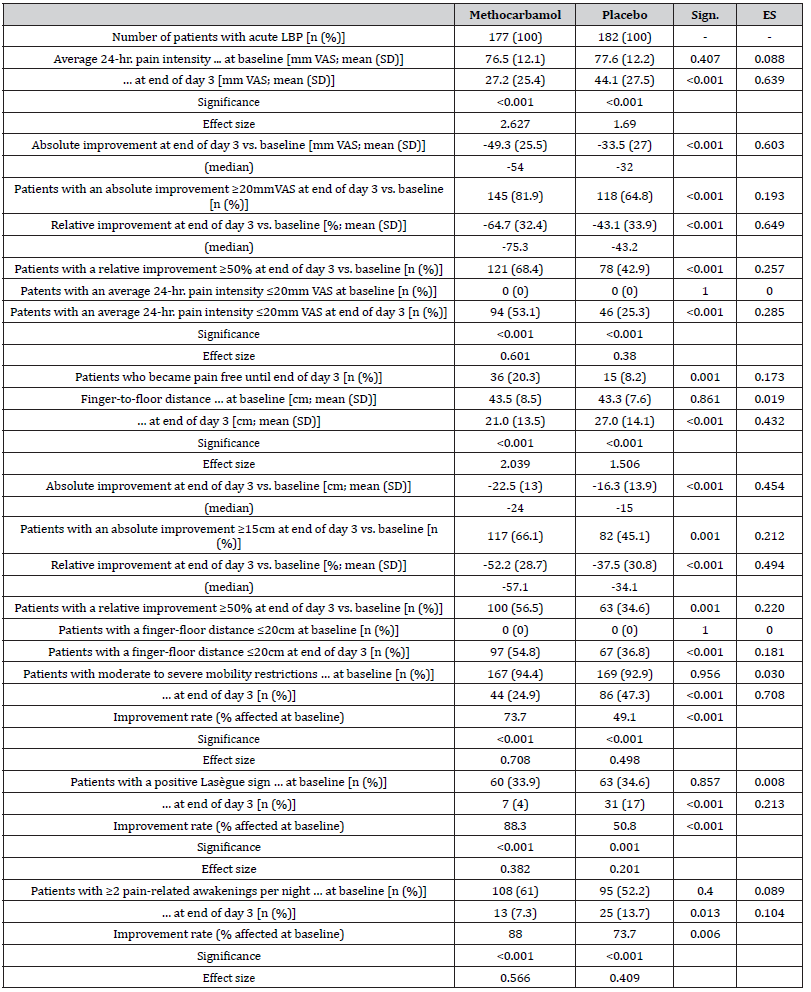
(Table 2) gives an overview over the relevant aLBP-associated parameters (e.g., pain intensity, finger-to- floor distance, mobility, Lasègue sign and interference with night sleep) at baseline and end of day 3 (incl. absolute and relative changes), as well as aggregated information on response rates and reasons for premature treatment discontinuations for both treatment cohorts.
Table 2:Acute (low) back pain characteristics at baseline and end of day 3 with treatment Abbreviations used: LBP: low back pain; n: number of patients; % percent; Sign.: significance; ES: effect size; hr.: hour; mm: millimeter; VAS: visual analogue scale; SD: standard deviation.
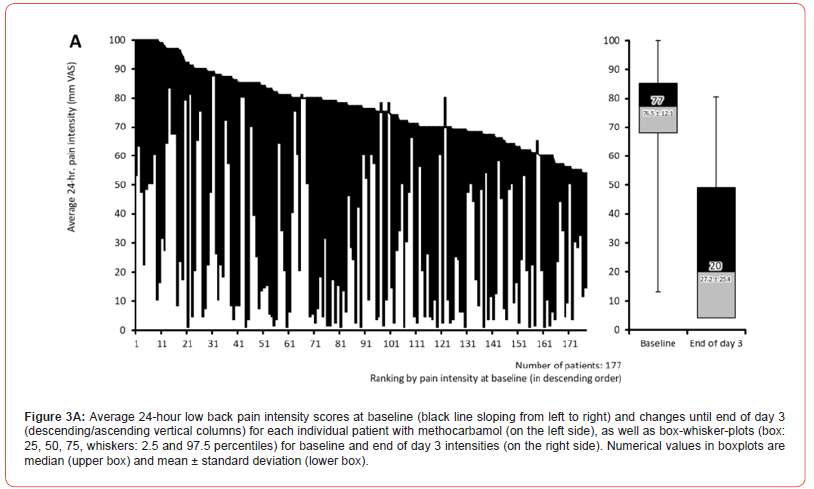
Pain intensity (PI)
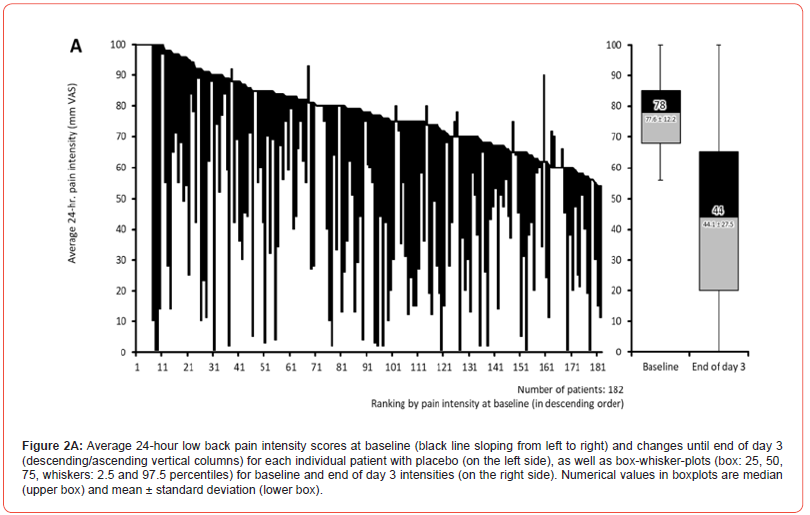
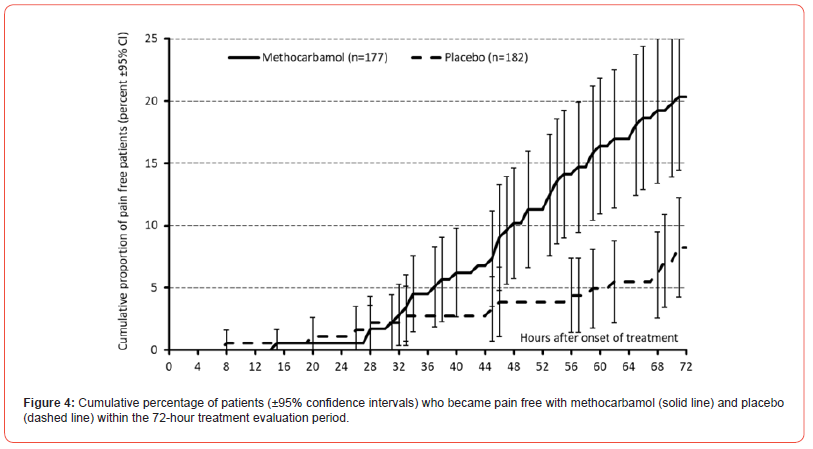
Pain intensity prior treatment with MET/PLC was 76.5±12.1 / 77.6±12.2 mm VAS (p=0.407) and improved significantly with both treatments until the end of day 1 compared to baseline: 58.4±18.6 / 63.5±17.7 mm VAS (p=0.009; ES: 0.279). At end of day 3, reported pain intensities for MET/PLC were 27.2±25.4 / 41±27.5 mm VAS (p<0.001, ES: 0.639). Absolute improvements for MET/PLC were - 49.3±25.5 / -33.5±27.0 mm VAS (p<0.001; ES: 0.603) and corresponding relative improvements were - 64.7±32.4 / -43.1±33.9 percent vs. baseline (p<0.001; ES: 0.649) (Figures 2 & 3). Percentages of patients who reported a clinically relevant absolute pain relief ≤-20 mm VAS vs. baseline were 81.9/64.8% for MET/PLC (p<0.001, ES: 0.193, those for clinically relevant relative pain intensity relief ≤-50% vs. baseline were 68.4/42.9% (p<0.001; ES: 0.257). Significantly more patients with MET (n=36, 20.3%) vs. PLC (n=15, 8.2%) became completely pain free (p=0.001; ES: 0.173) (Figure 4).
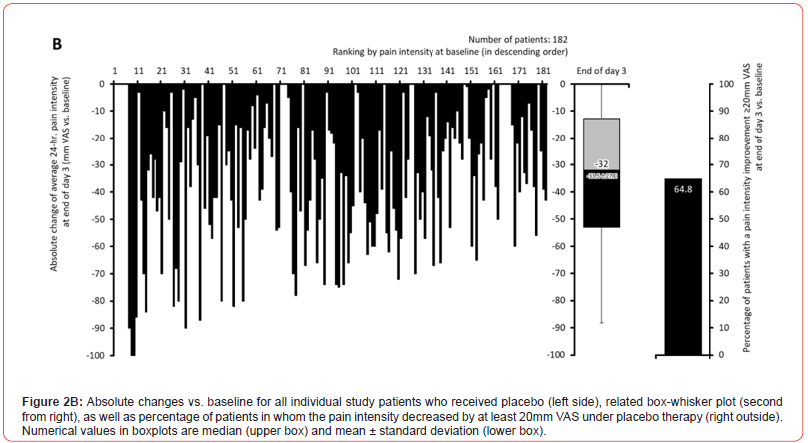
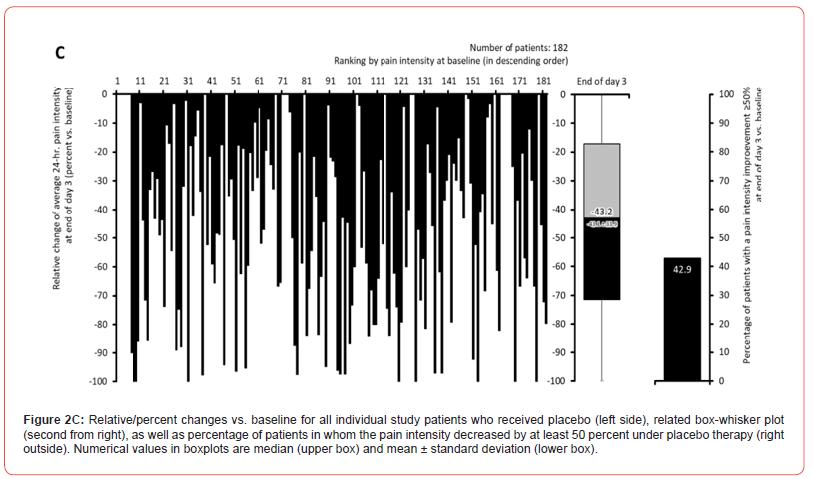
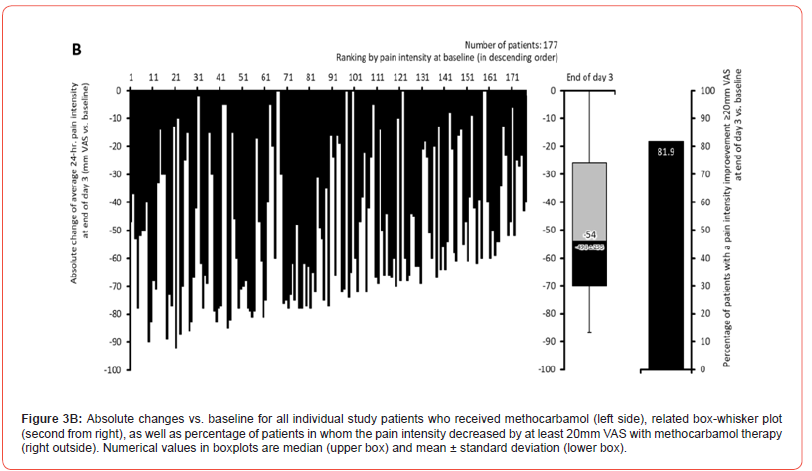
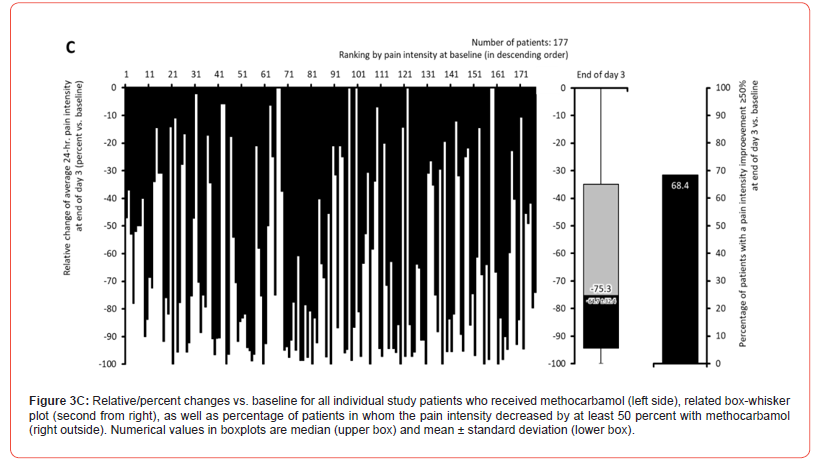
Finger-to-floor distance (FFD)
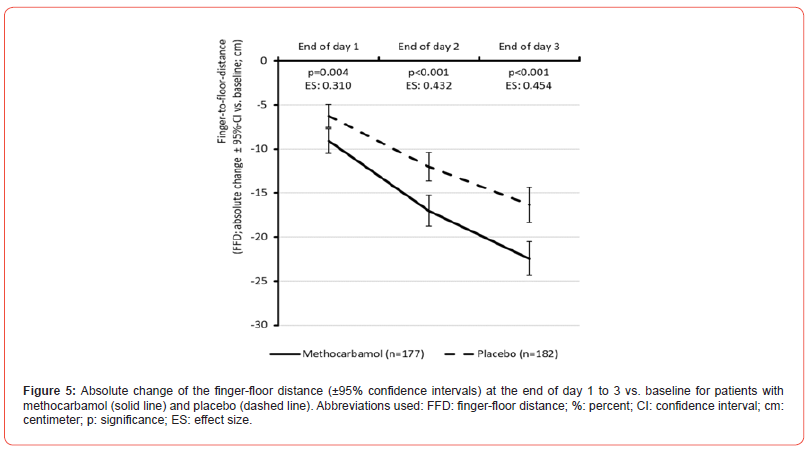
The FFD at baseline was 43.5±8.5 / 43.3±7.6 cm for MET/PLC (p=0.861). (Figure 5) shows that the evident improvement in FFD compared to baseline findings was not only already detectable at the end of day 1 of treatment with MET but was also significantly greater than that observed with PLC (p<0.001). Administrationrelated difference further increased and reached its maximum at the end of day 3 with MET/PLC: 21.0±13.5 / 27.0±14.1 cm (p<0.001; ES: 0.432). Absolute FFD improvement compared to baseline was -22.5±13.0 / -16.3±13.9 (median: -24 vs. -15) cm (p<0.001; ES: 0.454) and relative change was -52.2±28.7 / -37.5±30.8 percent (p<0.001; ES: 0.494). With 54.8% vs. 36.8%, significantly more patients with MET vs. PLC documented a FFD of 20 cm (or less) at end of day 3 (p<0.001; ES: 0.181).
Pain-related mobility restrictions (MOB)
Moderate to severe mobility restrictions were reported by 94.4/92.9% of MET/PLC-patients at baseline (p=0.956), and by 24.9/47.3% at end of day 3 (p<0,001; ES: 0.708). Improvement rates of those patients affected at baseline were 73.7/49.1% with MET/PLC (p<0.001)
Lasègue sign (LS)
A positive Lasègue sign – as a clinical correlate of painassociated movement restriction – was documented in 33.9/34.6% of MET/PLC patients at baseline (p=0.857) and 4.0/17.0% at end of day 3 (p<0.001; ES: 0.213). Improvement rates of those patients with a positive LS at baseline were 88.3/50.8% in response to the treatment with MET/PLC (p<0.001)
Pain-related sleep disturbances
Two or even more pain-related awakenings per night were reported by 61.0/52.2% of MET/PLC- patients at baseline (p=0.400) and only 7.3/13.7% at end of day 3 (p=0.013; ES: 0.104). Improvement rates of those patients affected at baseline were 88.0/73.7% following treatment with MET/PLC (p=0.006).
Physical limitations
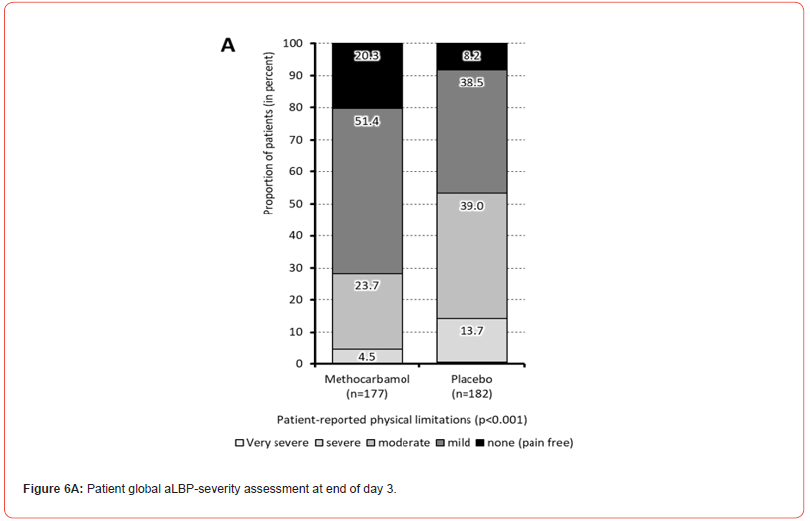
At baseline, the majority of patients treated with MET/PLC reported either very severe (n=106/102, 59.9/56.0%) or severe physical limitations due to their aLBP (n=67/74, 37.9/40.7%). During treatment, patients in both treatment groups (MET/PLC) showed a significant improvement of their physical impairments, which resulted in only n=8/25 (4.5/14.2%) with severe or very severe, n=42/71 (23.7/39.0%) with moderate and n=127/85 (71.8/46.7%) with mild or even no physical impairment at the end of day 3 with treatment (p<0.001; ES: 0.255) (Figure 6a).
Global effectiveness of study medication
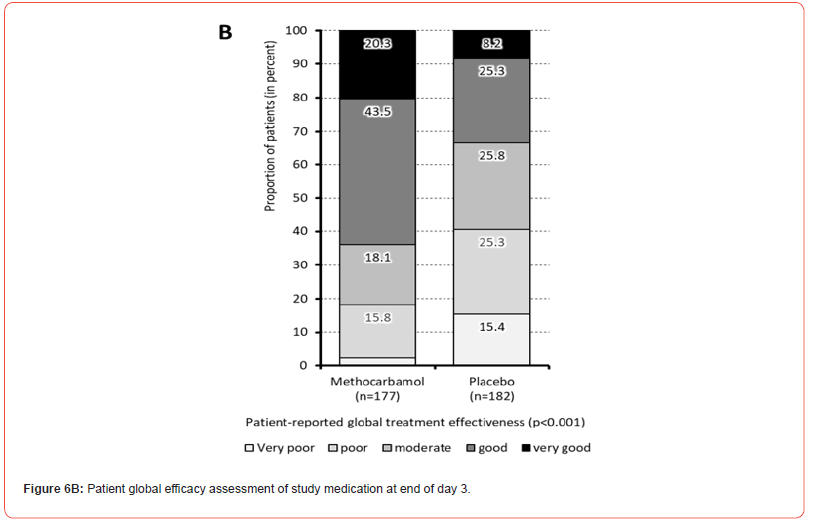
In the global efficacy assessment at the end of day 3 with treatment, 63.8/33.5% (n=113/61) of patients with MET/PLC rated their respective therapy as “very good” or “good”, 18.1/25.8% (n=32/47) as “moderate”, and 18.1/40.7% (n=32/74) as “poor” or even “very poor” (Figure 6b).
Safety and tolerability
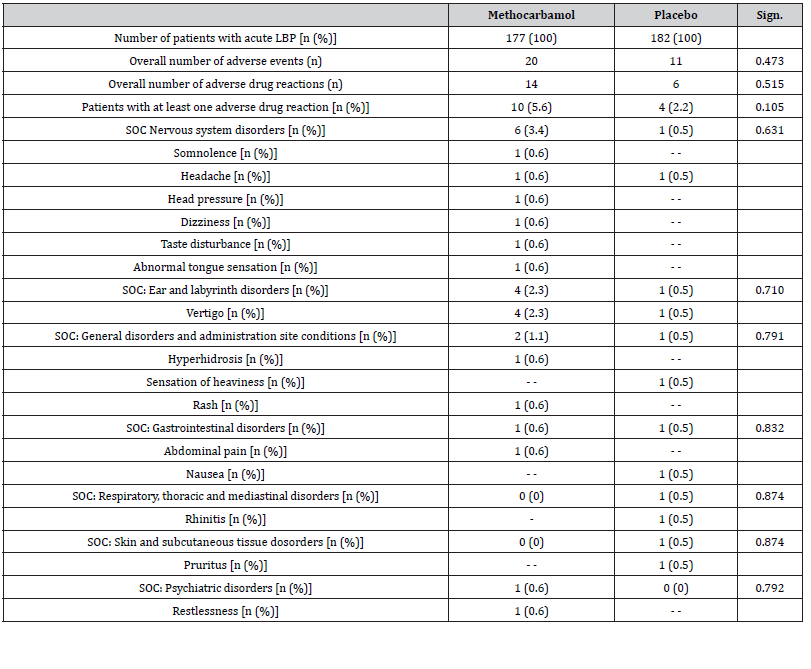
During the 3-day evaluation period, patients who received MET/PLC documented 20/11 AEs. Of those, 14/6 were classified as ADRs and occurred in n=10/4 study patients (5.6/2.2%; p=0.105) (Table 3). Most prevalent ADRs were related to the nervous system (n=6/1) and the vestibular organs (n=4/1). There was no evidence of a statistically significant above-random accumulation of ADRs due to the use of MET for any organ system. Insofar as the original study records contained information on this, there was also no evidence for any persistent or serious damage. Only in one case (with PLC) therapy was terminated prematurely as a consequence of the ADR. All other undesirable drug reactions documented resolved spontaneously and completely despite continuation of the study medication.
Table 3:Safety and tolerability overview. Abbreviations used: LBP: low back pain; n: number of patients; % percent; Sign.: significance; SOC: system of organ classes.
Primary endpoint
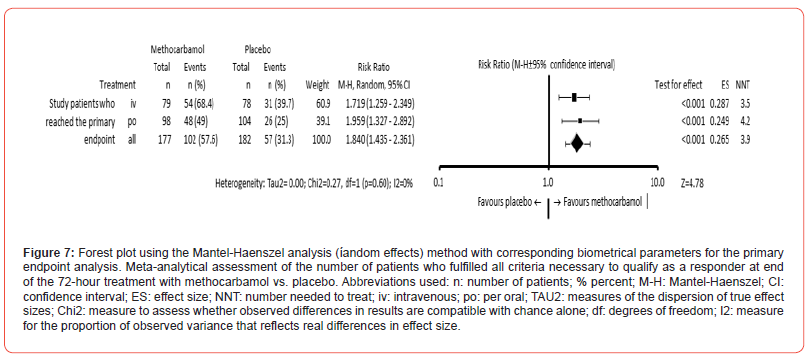
(Table 4) summarizes the data necessary for the assessment of the primary composite endpoint of this study. MET showed a significantly greater efficacy than PLC in all 5 parameters. On average, patients treated with MET/PLC achieved 3.9±1.5/2.9±1.8 endpoints (p<0.001; ES: 0.597). All 5 endpoints were achieved by n=102/57 patients (57.6/31.3%) treated with MET/PLC and classified them accordingly as responders (p<0.001; ES: 0.265; NNT: 3.9; OR: 2.982 (95%-CI: 1.935-4.596), RR: 1.840 (95%-CI: 1.435- 2.361); (Figure 7). Since a) the 95% CI for the aforementioned responder analysis (with 52.4-62.8% for MET and 26.5-36.1% for PLC) did not overlap, and b) the lower limit of the 95% confidence interval for MET (at 52.4%) was clearly above the upper limit for PLC (at 36.1%), and c) the effect size for this difference was (with 0.265) greater than the predefined cut-off value of 0.2, and d) the 72-hour discontinuation rate due to ADR, an insufficient analgesic effect or patient request was for MET (with n=21, 11.9%) significantly lower than those for PLC (with n=58, 31.9%; p<0.001), the treatment with MET did not only fulfil all requirements for a significantly stronger analgesic effect, but also for a significantly superior efficiency compared to PLC.
Table 4:Primary endpoint analysis for methocarbamol vs. placebo Abbreviations used: LBP: low back pain; n: number of patients; % percent; Sign.: significance; ES: effect size; mm: millimeter; VAS: visual analogue scale; cm: centimeter; CI: confidence interval; ADRs: adverse drug reactions.

Efficacy of MET depending on the mode of administration
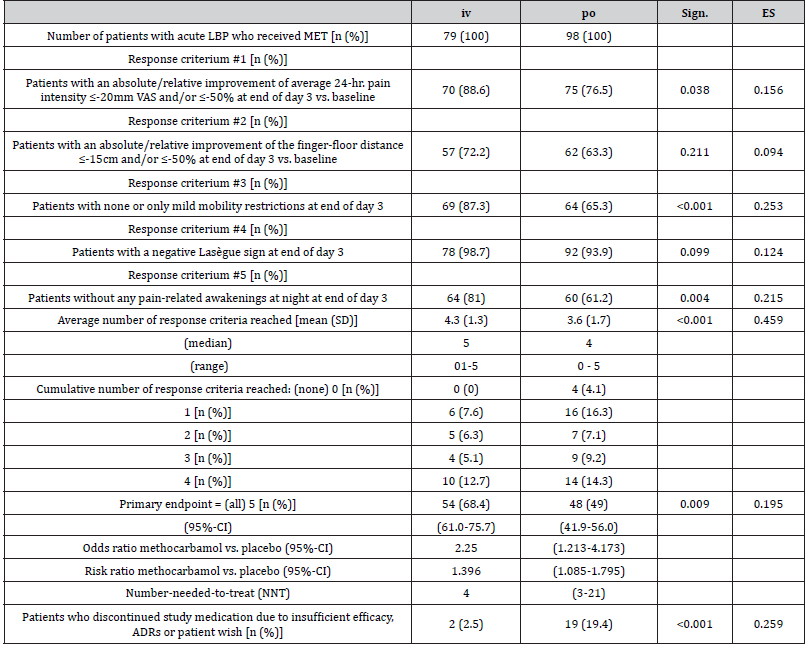
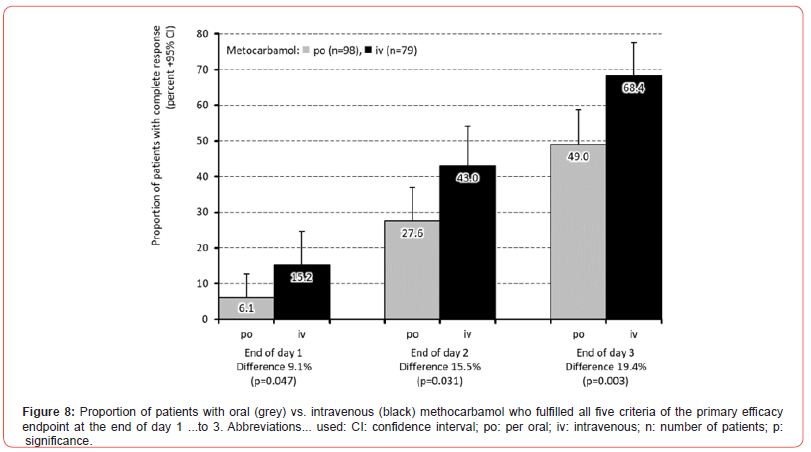
Table 5 outlines the main results of the efficacy and tolerability evaluation of MET depending on the administration form (iv vs. po). Statistically significant differences between iv/po were found at the end of the 3rd day of treatment with regard to the proportions of patients with no or mild restrictions in mobility (87.3/65.3%; p<0.001), for those without pain-related awakenings at night (81.0/61.2%; p=0.004) and for those who reported significant absolute and/or relative pain relief vs. baseline (88.6/76.5%; p=0.038). However, there were only insignificant differences with regard to the FFD improvement and the presence of the Lasègue sign. All in all, 2.5/19.4% of patients treated with iv/po MET discontinued their study medication prematurely either due to its insufficient analgesic effect, ADRs and/or patient request (p<0.001). At the same time, patients documented a mean response to MET iv vs. po with respect to 4.3±1.3 vs. 3.6±1.7 efficacy parameters (p<0.001), while 68.4% (95%-CI: 61.0-75.7%) vs. 49.0% (95%- CI: 41.9-56.0) reported a complete response in all five efficacy endpoints (p=0.009). Despite this significantly stronger effect and the lower treatment discontinuation rate, no superiority in efficacy could be formally demonstrated for the iv application of MET compared to the po administration in this analysis, as the effect size for the percent difference of patients with a complete response was (with 0.195) lower than the pre-defined threshold of 0.2. Looking at the time course of the effectiveness differences between the two dosage forms, the proportion of patients with a response in all five parameters of the primary efficacy endpoint of this study showed a significant between-cohort difference of 9.1% in favor of MET iv vs. po (15.2 vs. 6.1%; p=0.047) already at the end of the first treatment day, which further increased to 15.5% (43.0 vs. 27.6%; p=0.031) at the end of day 2 and finally to 19.4% (68.4 vs. 49.0%; p=0.003) at the end of day 3 (Figure 8).
Table 5:Primary endpoint analysis for iv vs. po methocarbamol Abbreviations used: LBP: low back pain; MET: methocarbamol; n: number of patients; % percent; Sign.: significance; ES: effect size; mm: millimeter; VAS: visual analogue scale; cm: centimeter; CI: confidence interval; ADRs: adverse drug reactions.
The results of this patient-level pooled re-analysis of two double-blind randomized controlled trials reported here on the efficacy and tolerability of a short-term treatment of aLBP with (either iv- or po- administered) MET did not only confirm the analyses of the original studies, but also provide additional (new) findings on the efficacy of this non-benzodiazepine and nonatropine- like antispasmodic. Among other things, we were able to demonstrate that the short-term use of MET over 72 hours not only led to significantly greater relief of aLBP-related pain and associated functional movement restrictions compared to placebo - with good tolerability - but also to a significantly greater reduction in pain-related impairment of the night sleep. Analyses of the time course of the parameters we evaluated also showed that these efficacy differences were already evident within the first 24 hours with a significantly higher proportion of patients who reported a complete response under MET (10.2%) compared to those under PLC (3.9%) in terms of the primary efficacy endpoint we defined for this study (p=0.0186).
In combination with an overall favorable side effect profile and the significantly lower proportion of patients who terminated their study treatment prematurely (either due to its insufficient analgesic effect, ADRs or other reasons of their own), the shortterm therapy with MET resulted not only in a significantly stronger analgesic effect, but also in a significantly superior effectiveness compared to PLC. These effects of the antispasmodic MET documented by us in this study are of particular importance, as the very early phase of aLBP in particular is not only characterized by a high degree of spontaneous remission (which makes it difficult for analgesic agents in clinical studies to prove a placebo-superior effect beyond doubt), but often also determines the extent to which those affected can exert a positive influence on a possible threat of chronification through own active measures. The possibility of using an appropriate therapy to alleviate increased muscle tone and associated movement restrictions in aLBP to such an extent that those affected can carry out physiotherapeutic measures necessary to normalize mobility is decisive for the prevention of chronification. Against this background, well-tolerated and effective antispasmodics - such as MET - play a special role as they are neither associated with serious central nervous system side effects (frequently seen with benzodiazepine-like myotonolytics) nor with the risk of autonomic and/or cardiovascular ADRs (related to the use of atropine-like acetylcholine receptor antagonists).
Subsequent analyses of the differential effects of intravenous vs. orally administered MET confirmed a comparable tolerability profile and provided significant evidence that the parenteral shortterm treatment led to a stronger and, above all, faster onset of analgesic effects compared with the conventional oral medication. Starting from 9.1% for day 1, the intergroup differences observed with regard to the responder frequency following iv vs. po MET decreased continuously with each subsequent day of treatment (to 6.4% for the second day and finally 3.9% for the third day), so that it can be assumed that the higher initial plasma concentrations after iv administration (due to the higher bioavailability and shorter time to peak plasma concentrations), particularly in the early phase of treatment, were probably responsible for this and that clinical relevance of these two pharmacological effects decreased continuously with increasing treatment duration. An observation that may serve as a starting point for the rational development of sequential combination therapies with MET – e.g., initiated with short-term iv treatment for 1-3 days, followed by an oral maintenance treatment for a further 7-14 days (e.g., to ensure the sustainability of the initially achieved treatment effects and to assist the implementation of physiotherapeutic non-drug treatment concepts).
In summary, these results are consistent with the reports of recent meta-analyses on the efficacy and tolerability of muscle relaxants in aLBP [15, 16]. However, it should be noted here that we have evaluated the short-term effects of a therapy with MET over a maximum duration of 72 hours in our analysis, while in the aforementioned network analyses the effects of mediumterm therapies with a duration of 4-6 weeks have been evaluated. Especially in the (very) early phase of aLBP, there is a need for scientific evidence regarding the benefit-risk profile of muscle tone-altering drugs that has not yet been covered by double-blind randomized studies. From a pain medicine perspective, this initial phase of symptoms is of particular importance, as those affected are shaped here on the basis of their individual experiences with regard to their later behavior and how they deal with movement-induced pain. In our view, the availability of an analgesic therapy with few side effects, which is also capable of quickly and significantly alleviating the functional limitations caused by aLBP due to its special mechanism of action, offers not only those affected the chance for a rapid normalization of symptoms, but also perspectives for a risk reduction of recurrent or even chronic courses (even if there is currently still no definitive proof of this hypothesis in form of high-quality placebo-controlled clinical trials).
Strengths And Weaknesses
The main strength of the present analysis is that the original data on efficacy and tolerability of MET that we´ve used for our re-analysis, have been collected in two independent, double-blind randomized, placebo-controlled clinical trials. Another one is that the aggregated evaluation of the newly extracted raw data at the patient level followed a confirmatory responder analysis based on a composite of five different endpoints – each of them relevant to everyday life. Within this responder analysis, MET showed significantly better results for each of the 5 individual response parameters and consequently also for the aggregated primary efficacy endpoint. Due to the specific mode of action and the short duration of use of MET, the frequency and severity of adverse drug reactions were limited. A constellation from which – in connection with the reported effects – concrete recommendations can be derived for daily practice.
Main weakness of this analysis is that the two clinical studies on which this evaluation is based have not yet been published or have only been published to a limited extent (only nationally in German) and thus made not really transparently comprehensible, which also explains why these data have not been taken into account so far in currently valid national and international guidelines or comparable official recommendations for the treatment of aLBP and other types of myofascial pain.
Acknowledgement
None.
Conflict of Interest
No Conflict of Interest.
References
- Chen S, Chen M, Wu X, Lin S, Tao C, et al. (2022) regional, and national burden of low back pain 1990–2019: A systematic analysis of the Global Burden of Disease study 2019. Journal of Orthopaedic Translation 10(32): 49-58.
- GBD 2021 Low Back Pain Collaborators (2023) 1990–2020, its attributable risk factors, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol 5(6): 316-329
- Menezes Costa LC, Maher CG, Hancock MJ, McAuley JH, Herbert RD, et al. (2012) The prognosis of acute and persistent low-back pain: a meta-analysis. Can Med Assoc J 184(11): E613-E624.
- Hoy D, Brooks P, Blyth F, Buchbinder R (2010) The epidemiology of low back pain. Best Pract Res: Clin Rheumatol 24(6): 769-781.
- McIntosh G, Hall H (2011) Low back pain (acute). BMJ Clin Evid.
- Hallegraeff JM, Krijnen WP, van der Schans CP, de Greef MHG (2012) Expectations about recovery from acute non-specific low back pain predict absence from usual work due to chronic low back pain: a systematic review. J Physiother 58(3): 165-172.
- Oliveira CB, Maher CG, Pinto RZ (2018) Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J 27(11): 2791-2803.
- Maher C, Underwood M, Buchbinder R (2017) Non-specific low back pain. Lancet 389(10070): 736-747.
- Zhang Y, Otto P, Qin L (2020). Methocarbamol blocks muscular Nav1.4 channels and decreases isometric force of mouse muscles. Muscle & Nerve 63(1): 141-150.
- Überall MA, Emrich OMD, Müller-Schwefe GHH (2017) Real-life efficacy and tolerability of methocarbamol in patients suffering from refractory muscle-related low/back pain - results of a health care research project based on data from the German pain practice registry. MMW Fortschr Med 159(7): 6-17.
- Emrich OM, Milachowski KA, Strohmeier M (2015) Methocarbamol in acute low back pain. A randomized double-blind controlled study. MMW Fortschr Med 157(5): 9-16.
- Überall MA, Küster MA, Müller-Schwefe GHH (2023) Efficacy and tolerability of intravenous methocarbamol in the short-term treatment of acute low back pain. Results of a double-blind randomised placebo-controlled multicentre study with 157 patients. Schmerzmedizin 39(1): 58-65.
- (2020) The Cochrane Collaboration.
- (2023) MS Office 365, version 2310.
- Jiang J, Pan H, Chen H, Song L, Wang Y, et al. (2022) Comparative efficacy of pharmacological therapies for low back pain: a Bayesian network analysis. Front Pharmacol 13: 811962.
- Wewege MA, Bagg MK, Jones MD, Ferraro MC, Cashin AG, et al. (2023) Comparative effectiveness and safety of analgesic medicines for adults with acute non-specific low back pain: systematic review and network meta-analysis. BMJ 380: e072962.
-
Michael A Überall*, Michael A Küster, Philipp Müller-Schwefe and Gerhard H H Müller Schwefe. Short-Term Efficay and Tolerability of The Antispasmodic Methocarbamol in Acute Low Back Pain – Results of Metabo, A Patient-Level Pooled Re-Analysis of Original Data from Two Double-Blind Randomized and Placebo-Controlled Trials. Arch Phar & Pharmacol Res. 3(5): 2023. APPR.MS.ID.000574.
-
Vaccinated people, Virus, Viral vectors, DNA molecules, SARS-CoV-2 virus, COVID-19
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






